Kampala, Uganda | THE INDEPENDENT | A committee instituted by the Ministry of Science, Technology, and Innovation says they lack sufficient pre-clinical study reports on Covilyce-1.
The committee from the Covid-19 Natural Therapeutics Clinical Trial School (CONAT) at Mulago Lung Institute was established to coordinate the scientific evaluation of traditional remedies innovated by local scientists and researchers for Covid-19 treatment.
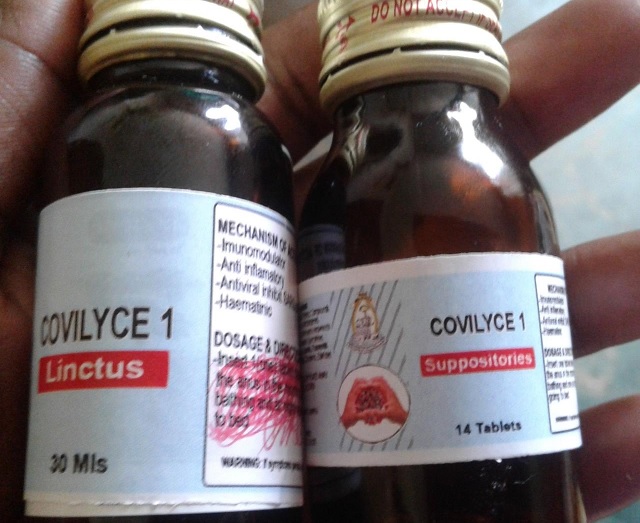
Covilyce-1, invented last year by lead researcher Dr. Alice Veronica Lamwaka, caught the attention of President Yoweri Museveni who authorized the release of 3.7 billion shillings to facilitate its research and clinical trials.
Whereas Dr. Lamwaka says her product is now set for clinical trials owing to anecdotal evidence of clearing Covid-19 symptoms in patients within 12 to 72 hours, members of CONAT however say no such reports have been availed to them.
Clinical trials refer to research studies performed in people that are aimed at evaluating a medical, surgical, or behavioral intervention and help researchers find out if a new treatment like a new drug or diet or medical device is safe and effective in people.
Dr. Moses Ocan, lecturer of Pharmacology at Makerere University and a member of CONAT says Dr. Lamwaka is yet to make a presentation on her product before a greenlight for clinical trials is given.
Dr. Ocan says once an innovator informs the ministry about the discovery of their product, they are referred to the CONAT team to provide sufficient information about the product which is later assessed before a decision is made to undergo human trials.
According to Dr. Ocan, so far the CONAT team has assessed five innovators out of which three gave sufficient information while two others had insufficient information about their products.
He says the three products which he didn’t reveal their details have been cleared for clinical trial tests.
Dr. Ocan however suspects that Dr. Lamwaka just like other innovators in the country is afraid to come forward over the contentious issues of patency and intellectual property rights on their invention.
He says although the matter is being handled at the Uganda Registration Board, many fear losing the rights to their products since the government will be the one funding the bulk of its research and clinical trial tests.
Dr. Ocan says Covilyce-1 herbal product can only advance to the next stage of clinical trials once the lead innovator provides sufficient preclinical study information on the product.
Dr. Lamwaka however says she is green about the CONAT team adding that she had no idea researchers were to go through them for assessment and evaluation.
She says whereas the team requires preclinical trial information, the team at the University is cash strapped after waiting in vain for money from the government which was to aid parts of the preclinical study.
“Like now we need to do the crude extract, give them the report, do the refined extract on what is exactly the cause. And now we can’t because we haven’t got that money, so we haven’t replied to them and besides that, the cost for paying for the clinical trial is too high for me to afford,” says Dr. Lamwaka.
Dr. Lamwaka also notes that the University should be given the freedom to explore, conduct teaching and research and hold community engagement on the innovation.
“We need to look for our intellectual partner and partner with them and work with them because this (condition) is too tying and we don’t even know about. I’m even hearing about it for the first time,” Dr. Lamwaka reacted to the conditions of the new committee.
Already, the health ministry is conducting clinical trials on the country’s first Covid-19 vaccine, UBV-01N developed by local scientists.
Brenda Nakzibwe, the Program Coordinator Presidential Science Initiative on Epidemics (PRESIDE) says the government is expected to release the results of the trials soon.
Last year, National Drug Authority(NDA) gave a go-ahead for the production of Covilyce-1 as a supportive treatment for Covid-19 pending clinical trial tests.
*****
URN
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price


